 Medical device company iThera Medical, our coordinator, announces that the fifth site has been initiated for the ongoing clinical study in the EUPHORIA project that aims to further develop the company’s proprietary optoacoustic imaging technology and demonstrate its clinical value in the assessment of inflammatory bowel disease (IBD).
Medical device company iThera Medical, our coordinator, announces that the fifth site has been initiated for the ongoing clinical study in the EUPHORIA project that aims to further develop the company’s proprietary optoacoustic imaging technology and demonstrate its clinical value in the assessment of inflammatory bowel disease (IBD).
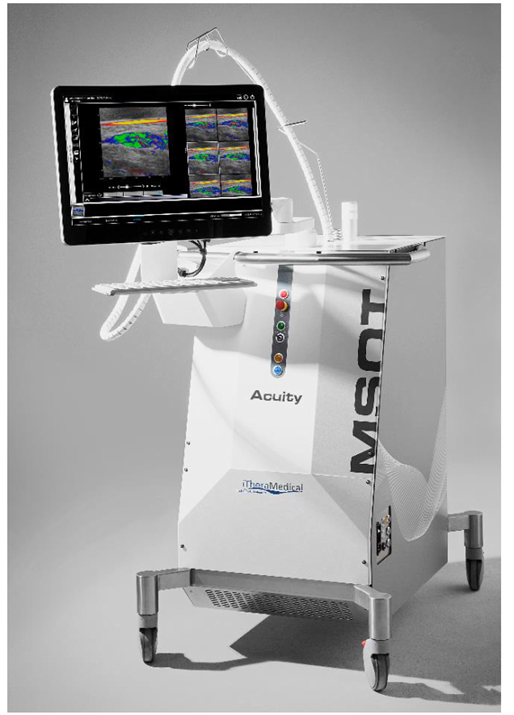
The EUPHORIA (Enhancing Ultrasound and PHOtoacoustics for Recognition of Intestinal Abnormalities) project aims to assess the ability of MSOT to measure disease activity in patients with IBD. Clinical validation of the diagnostic performance of the MSOT Acuity Echo investigational device is underway in a multicenter international study, with the I.R.C.C.S. San Raffaele Hospital (HSR) in Milan now added as the latest initiated site and the second site in Italy.
The initiation of new study sites will allow for faster patient recruitment to acquire data necessary for the clinical validation of the MSOT technology in the application of disease monitoring in IBD. Furthermore, iThera Medical will use the feedback from the various patient and investigator groups to guide the ongoing development of the MSOT technology to best benefit doctors and their patients.
Dr Mariangela Allocca – a leading physician at HSR’s Department of Gastroenterology and Gastrointestinal Endoscopy as well as EUPHORIA’s principal investigator at the Milan study site – comments on the site’s participation in EUPHORIA: “We are very excited about the initiation of HSR, and we hope to strongly contribute to the ongoing clinical validation for the use of MSOT technology in the non-invasive assessment of IBD.”
Read the official press release here.

